Avian Influenza: Kits compliant with the new regulations

Following the new regulations concerning the management of the spread of the Avian Influenza virus: Ensure the conformity and quality of your results with the new versions of our PCR kits!
Find out more about the new regulations for the Avian Influenza vaccination campaign:
Discover the new versions of our kits for the detection of Avian Influenza, validated by the National Reference Laboratory for Avian Influenza (ANSES, Ploufragan-Plouzané-Niort):
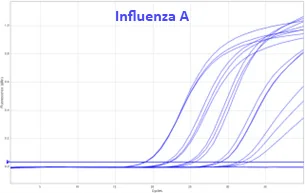
Bio-T kit Avian & Swine Influenza Virus V2
BIOTK139 (100 reactions)
Detection of Avian and Swine Influenza virus type A genomes by real-time RT-PCR with endogenous (IPC) and exogenous (IC) internal positive control.
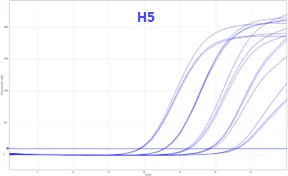
Bio-T kit AIV genotypes H5 & H7 V2
BIOTK139 (100 reactions)
Detection of Avian Influenza A virus genomes and H5 and H7 subtypes by real-time RT-PCR with endogenous (IPC) and exogenous (IC) internal positive control.
Benefits of our kits
.png)
New matrices validated: Chiffonnettes and pédichiffonnettes
.png)
To ensure the quality of your test results on all matrices, both kits feature two types of IPC: endogenous and exogenous
.png)
New endogenous IPC system ensures detection quality on all types of matrices and all poultry species
.png)
The kits have been validated on a wide range of samples from a variety of farmed and wild species
